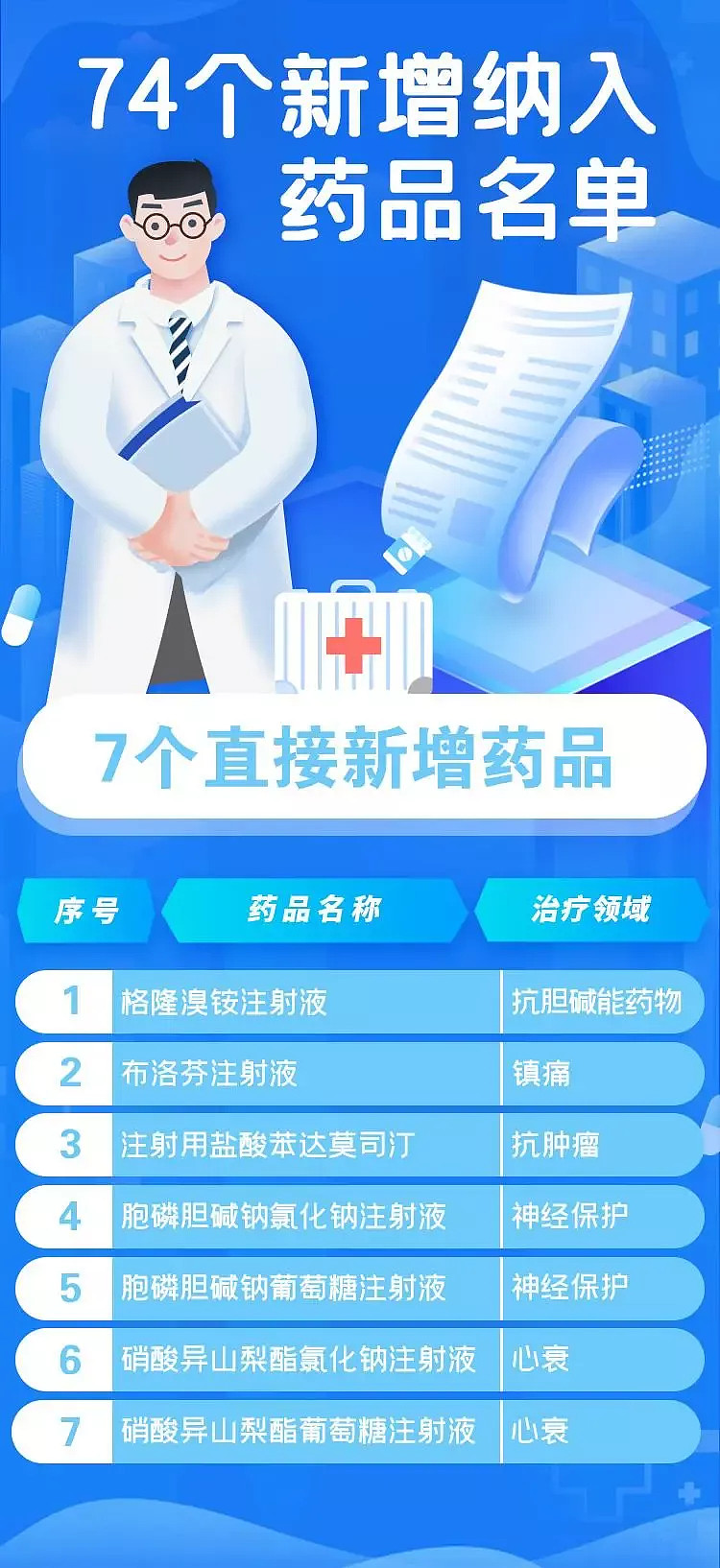
74 new medical insurance lists, including antibody drugs, ADC and gene therapy. …
On the 3rd, the medical insurance catalogue for 2021 was officially released-74 kinds of drugs were added and 11 kinds of drugs were transferred out, with a total of 2,860 kinds.
Among the 74 new drugs, It contains 18 anticancer drugs (bendamustine hydrochloride for injection, Donafenib tosylate tablets, Enshatini hydrochloride capsules, metformin mesylate tablets, dactinib tablets, obutinib tablets, otozumab injection, fluzopali capsules, Pampali capsules, Abexili tablets, Ailibulin mesylate injection, Nalatinib maleate tablets, Suo Fan tinib capsules and daretouzumab injection. Videcituzumab for injection), 7 kinds of drugs for rare diseases (human coagulation factor IX, etibant acetate injection, Nocicnatron sodium injection, aminopyridine sustained-release tablets, concentrated solution for Faaga glycosidase α injection, elouzumab injection, and chlorobenzoic acid soft capsules). (Photo details are attached at the end of the article)
It is not difficult to see that drugs such as antibodies, ADC and gene therapy have entered the medical insurance one after another, and bio-innovative drugs are no longer "high-priced drugs" that cannot be used. In addition to new drugs, the domestic PD-1/L1 that has entered the medical insurance has added new indications, and PK has dropped foreign imported products, and the spring of domestic bio-innovative drugs has already appeared.
01
All three new indications of domestic PD-1 were included.
Imported PD-1/L1 was completely annihilated.
In 2020, four domestic PD-1 products have been included in medical insurance, and this medical insurance negotiation mainly focuses on new indications. According to the latest results of medical insurance negotiations:
Cinda’s Cindilizumab added three indications: first-line squamous non-small cell lung cancer (NSCLC)+ first-line non-squamous NSCLC+ first-line liver cancer;
There are three new indications of "first-line squamous NSCLC+ first-line non-squamous NSCLC+ second-line liver cancer" in the temerilizumab of Baekje Shenzhou.
Junshi’s Treprilizumab added two indications: "third-line nasopharyngeal carcinoma+second-line urothelial carcinoma".
The two indications for nasopharyngeal carcinoma added by Hengrui’s Karelizumab this year have not entered.
At present, the prices have not been announced yet. Its medical insurance price in 2020 is 2180 yuan/piece (100 mg); Karelizumab 2928 yuan/piece (200 mg); Trepril monoclonal antibody 906 yuan/piece (80 mg); Cindilizumab 2843 yuan/piece (100mg).
On the import of PD-(L)1, Pabolizumab injection of Merck, Nivolumab of Bristol-Myers Squibb and Duvalizumab of AstraZeneca all passed the preliminary examination, but none of them entered the medical insurance. At the same time, Bristol-Myers Squibb was absent from the medical insurance negotiation site before, and the industry speculated that they were suspected of giving up.
In addition, Ampley monoclonal antibody of Kangfang Bio and Cypalimab of Yuheng Bio were approved in August this year, so they missed the medical insurance negotiation. The price of Ampley monoclonal antibody was 4,875 yuan /100mg, combined with the supporting drug donation policy, the average annual fee was 19,500 yuan; The price of Cepalimab is 3,300 yuan /120mg(4ml), and the annual treatment cost reaches 86,000 yuan. At present, the policy of giving medicine is not clear.
Generally speaking, after this round of medical insurance negotiations, the domestic PD-1 competition will be more intense as a whole.
02
ADC drugs:
Rongchang Xiti list, Takeda did not enter.
As the first domestic ADC drug, Rongchang Bio’s vidicon was included in the medical insurance for the treatment of third-line HER2 overexpression gastric cancer.
At present, there are three kinds of ADC drugs listed in China, including Emetizumab from Roche (trade name: Herselet), Vebtuximab from Takeda (trade name: Anshili) and Vidiximab from Rongchang Bio (trade name: Aidixi). In the preliminary list, only verbotezumab and vidiximab were shortlisted, and Emetizumab was not included in the preliminary list and was not listed in the 2020 medical insurance catalogue products.
It is reported that the annualized cost of Emetizumab, which is also the target of HER2, is still close to 600,000 after considering charitable donation, while the annualized cost of gastric cancer indications after the donation of Veditzumab is about 340,000. The medical insurance price of Veditzumab has not been announced yet, but it should also be greatly reduced.
In addition, it is worth mentioning that another new fusion protein product of Rongchang Bio-recombinant B lymphocyte stimulating factor (BLyS)/ proliferation-inducing ligand (APRIL) for injection for the treatment of systemic lupus erythematosus, Tetasip, was also included in the new medical insurance list this time, which indicates that China is at the forefront of the world in the research and development of new drugs for the treatment of systemic lupus erythematosus.
03
A variety of rare disease drugs are included in medical insurance.
700,000 SMA gene drugs dropped to 33,000.
On December 23, 2016, the world’s first SMA (spinal muscular dystrophy) precise targeted therapy drug-Nocicnatrium injection developed by Bojian Company was included in the new medical insurance list. It is reported that in April, 2019, Nocicna Sodium Injection was listed in China, and became the first SMA treatment drug in China. However, its treatment cost is close to 700,000 pieces per injection. Even if it participates in the patient assistance program, the average annual cost is about 1.05 million yuan, which is undoubtedly a huge amount for many ordinary families in China.
(Spinraza is an antisense oligonucleotide, which can change the splicing of SMN2 gene and increase the production of fully functional SMN protein, and belongs to a gene therapy drug. By intrathecal injection, Nocicnatrium injection can directly deliver the drug to cerebrospinal fluid around spinal cord, thus improving motor function, increasing survival rate and changing the disease process of SMA. This drug won the honor of "Best Biotechnology Product" in the 2018 International Prix Galien in November 2018.
In fact, since its listing in China in 2019, Nocicna sodium has been included in the agenda of medical insurance negotiations, but its high price does put tremendous pressure on medical insurance. However, in June this year, Roche’s SMA oral drug Risperidone entered the China market, and the retail price of its 60mg/ bottle was set at 63,800 yuan, and the annual cost after counting the donation project was 650,000 yuan. This has formed a strong competition for Nosema Natrium, which has reduced its annual fee to 550,000 yuan, so it is possible to enter the medical insurance negotiation.
It is reported that this time, Nocicna sodium was officially included in medical insurance, and it has gone through 8 negotiations and 9 quotations. The quotation of 5 ml and 12 micrograms per unit has dropped from 53,680 yuan to 33,000 yuan, a decrease of nearly 40% before and after.
04
Domestic drugs for Alzheimer’s disease are included in medical insurance.
In June this year, Aducanumab, Aβ(β (amyloid β-protein) antibody developed by Bojian Company for Alzheimer’s disease (AD), which was approved by FDA, was controversial. Six months later, China’s original new drug for the treatment of Alzheimer’s disease-Mannturner Capsule of Green Valley Pharmaceutical (trade name "Jiuqi No.1", code name GV-971) was included in medical insurance, and the price dropped from the original 895 yuan/box to 296 yuan/box, with a drop of over 60%.
05
1.2 million CAR-T cell therapy is not included in the list.
In addition to the good news, it is a pity that the first CAR-T cell therapy drug in China, which has attracted much attention, the 1.2 million yuan-worth of Akilensai injection (trade name: Yikaida) was selected into the List of Drugs that passed the preliminary examination in the 2021 national medical insurance drug list adjustment, and was qualified to enter the next adjustment link, but failed to enter the negotiation link in the end.